High Level Functionality
OpenELIS comprises two products; a laboratory component and a client web portal. The laboratory component supports the activities of laboratory staff and closely matches many of the testing and reporting processes. The web portal is designed to allow clients and customers to order tests electronically, access to their results, ability to sign-up for notifications, or to monitor the status of their samples. The web portal also offers a component for Public Health Department’s to access results statewide for reportable conditions and for Newborn Screening Follow-up. A third product is in development that will allow the LIMS Administrator and/or trained laboratory staff the ability to configure instrument interfaces that connect OpenELIS to multiple testing instruments.
The high-level functionality available through OpenELIS includes:
Domains for Clinical, Environmental, Animal, Proficiency Tests, and Newborn Screening with Follow-up (under development).
Login sample and analysis domain specific information into one system for overall efficiency in training staff.
Sample Management
Quick entry for fast logging in of samples; verification of sample/domain specific data; track sample from entry in the lab through resulting out of test result
Scan/attachments to eliminate paper
Easily scan and attach paper files throughout the specimen life cycle
Comprehensive test definition including result validation, prep and reflex rules, QC definition
LIMS Administrator and/or trained lab staff to define tests/results, QC, prep/reflex tests, worksheets, etc.
Electronic worksheet with QCs, batch result entry, export instrument orders and import instrument results
Worksheet templates can be defined to include QC, used to enter batch results for manual testing, to export to an instrument for orders or import instrument results.
Instrument Interface (under development)
LIMS Administrator and/or trained lab staff can configure an interface for each assay performed on an instrument that allows automated communication between the LIMS and the instrument for orders/results.
Specimen storage
Track specimen storage, move to new locations, and discarding the specimen.
Inventory Management with lot and expiration information for items, orders, kits, shipping
Track all lab inventory including lot and expiration date on items; create send-out orders for kits and ship them to the client for complete tracking of where kits are going.
Billing interface
Send accurate billing data to billing software by configuration of data elements.
Flexibility to collect additional demographic data
Easily collect additional sample/analysis data for public health reporting which can be configured by test.
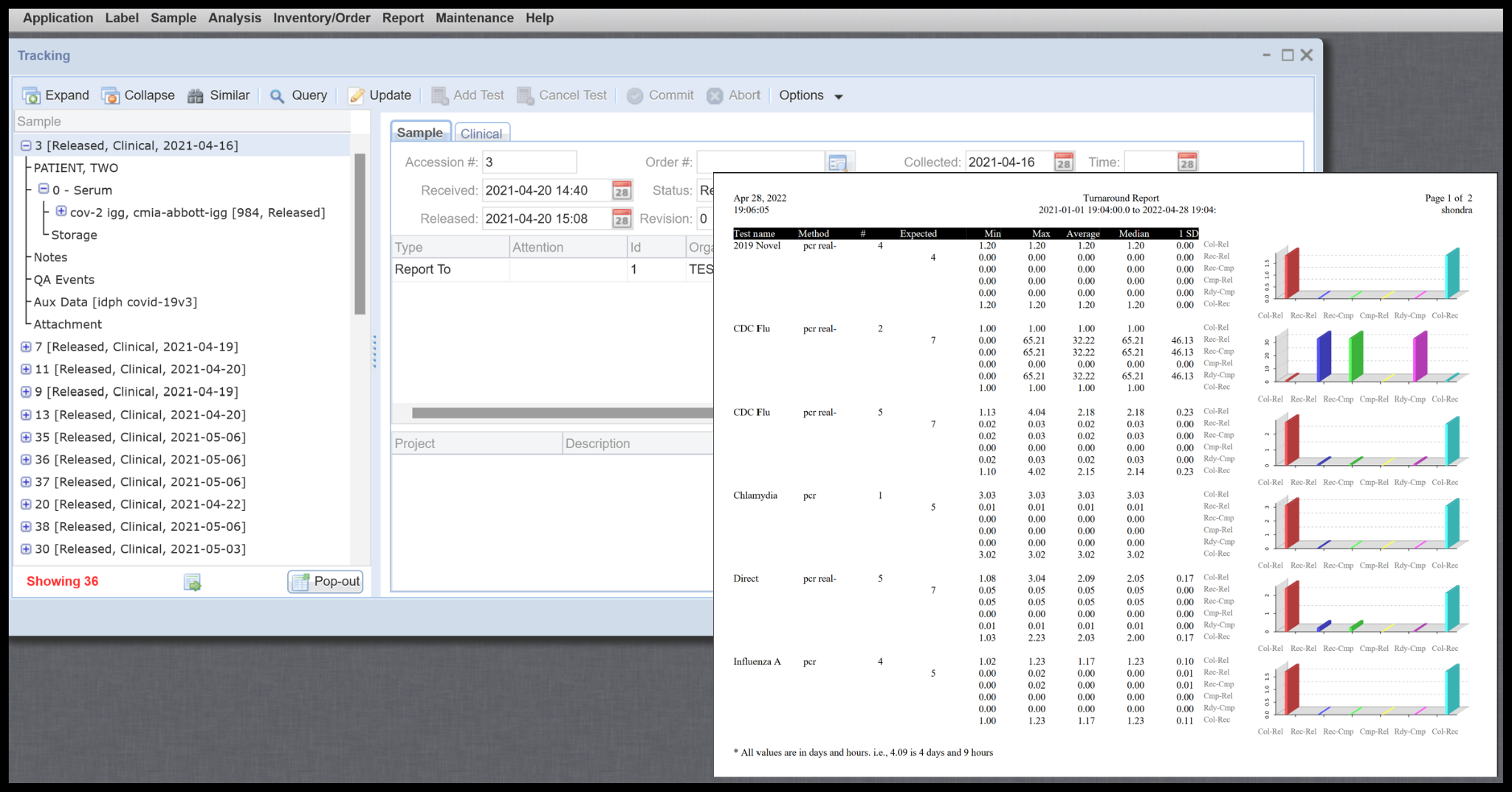
Management, QC plot, Turnaround, and selectable ad-hoc reports including Web Reports
Multiple management canned reports to easily access summary level data and means to create detailed queries to pull data for additional reporting needs.
User management with flexible permissions to modules and workgroups
LIMS Administrator and/or trained lab staff can configure users within the Security module assigning the appropriate access levels to LIMS users.